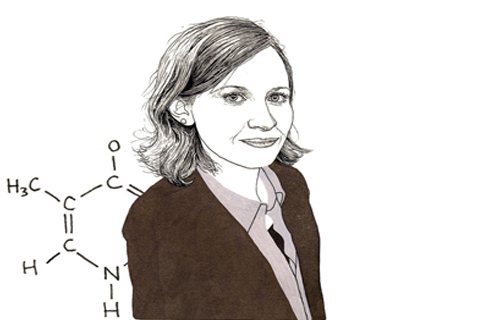
Julie Claycomb talks about her research into RNA interference the way a college football star might talk about getting drafted into the NFL – as though she’d trained her whole career to be the best at what she does, and now can’t quite believe she’s made the big leagues.
Claycomb discovered a love of lab science at MIT. In grad school and during a post-doctoral fellowship she was mentored by global leaders in molecular biology, including Nobel Laureate Craig Mello, who inspired her to devote her career to the field. Today, at 35, she’s helping to create a whole new playbook of therapies that might one day tackle genetic diseases before they ever have a chance to go on the offensive.
Claycomb studies a group of proteins known as Argonautes. These proteins could be powerful tools in the effort to pre-emptively disrupt damaging genetic processes. Her work challenges the very idea that DNA determines destiny.
A strand of human DNA is a blueprint containing instructions about everything from eye colour and hormone levels to predisposition to certain cancers. Claycomb, who is a professor of molecular genetics, researches how that genetic information gets turned into an actual living organism – and how that process can be controlled.
DNA is a type of nucleic acid. It copies itself to another type of nucleic acid molecule known as messenger RNA through a process called “transcription.” The messenger RNA molecules are then “translated” from the molecular language of nucleic acid into the proteins that are the building blocks of life.
Transcription and translation turn genetic information into bones and flesh, neurons and blood, eyelashes and teeth. They help the body defend against illness, but also make the organism vulnerable to genetic diseases. “There’s a lot we need to learn about how those processes work,” Claycomb says. “If we can understand the mechanisms regulating these processes, maybe we can exploit them to make more potent therapeutics.”
When it comes to running genetic interference, Argonaute proteins are a triple threat. “They can degrade messenger RNAs, recruit other RNAs that stop translation, or muck about with the DNA in the nucleus to stop the process of transcription,” she says. This means, for instance, Argonautes could “silence” a breast cancer gene, rendering it harmless to the woman who carries it.
RNA interference is already being clinically tested as a treatment for conditions such as macular degeneration and respiratory syncytial virus. (Viruses work by attacking the DNA in an organism’s cells. This makes them potential targets for RNA interference.)
Thousands of illnesses, from hepatitis to HIV-AIDS to Huntington’s disease might be mitigated with RNA interference research. “We’re reaching an age where we can do so much with the information that’s out there,” she says. “More and more people are learning how we might harness RNA interference research for therapeutics.”
Recent Posts
U of T’s 197th Birthday Quiz
Test your knowledge of all things U of T in honour of the university’s 197th anniversary on March 15!
Are Cold Plunges Good for You?
Research suggests they are, in three ways
Work Has Changed. So Have the Qualities of Good Leadership
Rapid shifts in everything from technology to employee expectations are pressuring leaders to constantly adapt