As Canada comes up on the one-year anniversary of its first reported case of COVID-19, two of the many vaccines under development around the world are on the verge of being approved for widespread use. But there are questions about how well these initial vaccines will work, how quickly they’ll become available, and who will get them.
That’s why a number of U of T scientists are working to develop their own vaccine candidates. They expect we will need more than one to bring the pandemic under control. And they believe that increasing the chances of a made-in-Canada vaccine will help to ensure that all Canadians can get protection.
“I think it would be great to have a vaccine developed by Canadians, made by Canadians and owned by Canadians,” says Robert Kozak, a clinical microbiologist at U of T and the Sunnybrook Health Sciences Centre, who is working on a vaccine. “That makes it easier to distribute, and to make sure it’s spread equitably – in Canada, and all over the world.”
About a dozen of the roughly 200 vaccines in development globally are in Phase 3 clinical trials, meaning some could be ready for manufacture early in 2021. Canada has already agreed to spend more than $1 billion reserving vaccines being developed by seven pharmaceutical companies. Only one of them, Medicago, is located in Canada.

Because there are so many ways to design a vaccine, it is difficult to tell from the beginning which ones will provide the most protection. It’s even possible that some vaccines will work better than others for certain populations – such as older people or children. The vaccines being developed at U of T aren’t likely to get out first, but they could easily find a place in the mix.
U of T scientists are pursuing several different approaches to creating a vaccine. These include using custom-designed DNA or RNA (ribonucleic acid); injecting antigen-producing genetic material into a cell; and even modifying a century-old tuberculosis vaccine so that it will fight COVID-19.
When the body is invaded by a new pathogen, such as the virus responsible for COVID-19, the immune system learns to recognize it by the molecular patterns on its surface. The next time that this pathogen shows up, the immune system is prepared to jump into action.
Vaccines teach our immune system to recognize the patterns on a pathogen ahead of time. Some use dead or weakened pathogens to do the job. Others use just a part of the virus – possibly a piece of one protein – to train the immune system.
A DNA Vaccine
Kozak is working with Gary Kobinger at Laval University on a DNA “ring” that carries the code for the SARS-CoV-2 spike antigen – a small piece of the virus responsible for COVID-19.
One of the normal jobs of our cells is to take instructions from DNA to produce proteins. Kozak and Kobinger designed a loop of DNA that they deliver into the body. When our cells’ protein-making machinery encounters this DNA, it carries out the instructions on it and makes the viral protein. However, once the viral protein is produced, the immune system still recognizes it as foreign and develops an immune response to it. “Our method will train the immune system so that when the immune system sees the real thing it’s going to be ready to fight it off,” says Kozak.
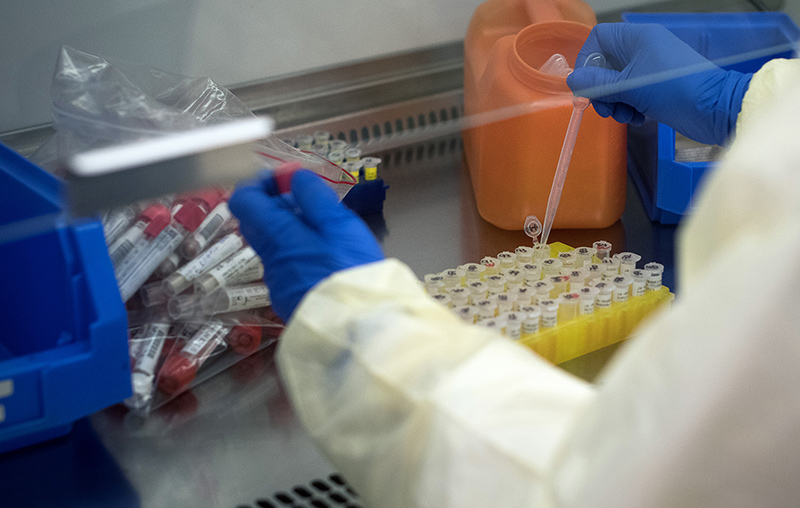
DNA vaccines are a newer technology, so no existing vaccines use it yet. Kozak says it is an attractive approach because the DNA loops are cheap and easy to make, they show promise in creating a good immune response and they are safe because no live virus is used.
The protein Kozak and Kobinger are using is called the spike protein. It’s part of the protrusion on the outside of the coronavirus. The spike protein is an attractive target because the virus needs it to attach to human cells and begin the infection process. Antibodies that target it will smother the spike protein and prevent it from infecting cells. In the meantime, the immune system ramps up to destroy the virus.
So far, Kozak and Kopinger have shown that their vaccine creates an immune response in animals. Now they are testing it to see if animals given the vaccine actually gain protection against illness. Although they are still analyzing results, Kozak says he’s cautiously optimistic the data will show that vaccinated animals get sick less than unvaccinated animals.
Using Messenger RNA
Mario Ostrowski is a U of T professor of immunology and an infectious disease clinician at St. Michael’s Hospital in Toronto. He is collaborating with a Toronto biotech company called Providence Therapeutics to create something similar to a DNA vaccine, except by using messenger RNA.
Messenger RNA is a single-stranded molecule that helps translate the information coded in DNA into a protein. Providence was using it to develop a cancer treatment before the pandemic, and Ostrowski was interested in adapting it for HIV. But when COVID-19 hit, Ostrowski and Providence pivoted. “The advantage of messenger RNA vaccines is you can produce these vaccines quite rapidly and really tailor and design them according to the proteins you want,” Ostrowski says.
In this case, the scientists are also getting the body’s cells to use the virus genetic code to manufacture a piece of the virus spike protein. The researchers have already shown that the vaccine provokes an immune response in animals, and they are moving ahead with studies to see if vaccinated animals show a resistance to infection with the virus.
Ostrowksi says they are also studying people who have already had COVID-19 to see what molecular patterns on the virus their immune systems learned to recognize. Those patterns could also be promising targets for a vaccine.
Tuberculosis and a Hollowed-out Virus
Jun Liu, a professor of molecular genetics, is working on a vaccine with two other U of T researchers – James Rini, a professor of molecular genetics, and Jim Hu, a professor of laboratory medicine and pathobiology, and a senior scientist at the Hospital for Sick Children. The team is also using the spike protein as an antigen. But they are developing two different vaccines, each with its own way of introducing the antigen into the body.
The first uses something called a helper-dependent adenovirus, or HDAd. These are viruses that have been “hollowed out.” All of their original genetic material has been deleted, allowing researchers to insert the genetic material they want. The HDAd still attaches to cells and injects the genetic material. But because its own genetic material has been deleted, it can’t make new viruses.
Although HDAds have been used in research and in gene therapy, they have not been used in any clinical applications before – including approved vaccines. Hu says they have the potential to create a potent and safe vaccine with few side effects.
The other approach the three researchers are pursuing begins with a very old vaccine called bacille Calmette-Guérin, which has been used against tuberculosis since 1921. Many researchers are interested in it because it seems to have a general immune-boosting effect that all by itself might offer some protection against COVID-19.

Liu’s team is modifying the bacterium used in bacille Calmette-Guérin so that it will create a protein that contains two different antigens – the original tuberculosis antigen, combined with the antigen of the spike protein in the virus responsible for COVID-19. The intention is that this protein will create the same general immune-boosting effect along with a specific immunity to COVID-19. “It’s difficult to predict which platform will be successful. We want to increase our chances,” Liu says.
The team received a $415,000 grant from the Canadian Institutes of Health Research to pursue the vaccines, and, like U of T’s other vaccine candidates, has already had some success with animal testing. But vaccine approval requires large clinical studies with humans – first to show that the vaccine is safe, and then to prove that it is effective. The cost can run into the tens of millions of dollars. “We need a lot of support to get it going,” says Hu.
All of the teams have received some government funding for their research, but they will need to attract additional funding, corporate sponsors, investors or some combination of these to develop the vaccines past the animal testing stage. With other countries making the investment already, and some vaccines close to coming to market, it can be hard to convince funders, the scientists say. But they think it is important for Canada to have its own vaccine efforts. “Many of the vaccines going through Phase 3 trials are being done in the U.S.,” Ostrowski says. “We don’t know when we’re going to have access to any of these vaccines.”
Ultimately, many vaccines will need to be approved before the pandemic is brought under control. The U.S. Food and Drug Administration has said it expects any COVID-19 vaccine it licenses will need to protect at least 50 per cent of people who take it. That would be a good start, but would still leave room for improvement. “If you want to achieve long-term protection, you might have to do a mix and match,” Kozak says. My opinion is you’ll see a lot of these different vaccines going forward.”
Recent Posts
People Worry That AI Will Replace Workers. But It Could Make Some More Productive
These scholars say artificial intelligence could help reduce income inequality
A Sentinel for Global Health
AI is promising a better – and faster – way to monitor the world for emerging medical threats
The Age of Deception
AI is generating a disinformation arms race. The window to stop it may be closing





2 Responses to “ The Search for a Vaccine ”
What is the date of this information?
@Margaret
The information for this article was gathered in October 2020.